Synthesis of Tylenol ® (Acetaminophen)
Background Information
Acetaminophen is a widely used over-the-counter medicine because it is a powerful analgesic (relieves pain) and antipyretic (reduced fever). It is commonly used for fever, headaches, as well as minor aches and pains. It is also relatively inexpensive, has a low toxicity (when used properly) and few side effects.
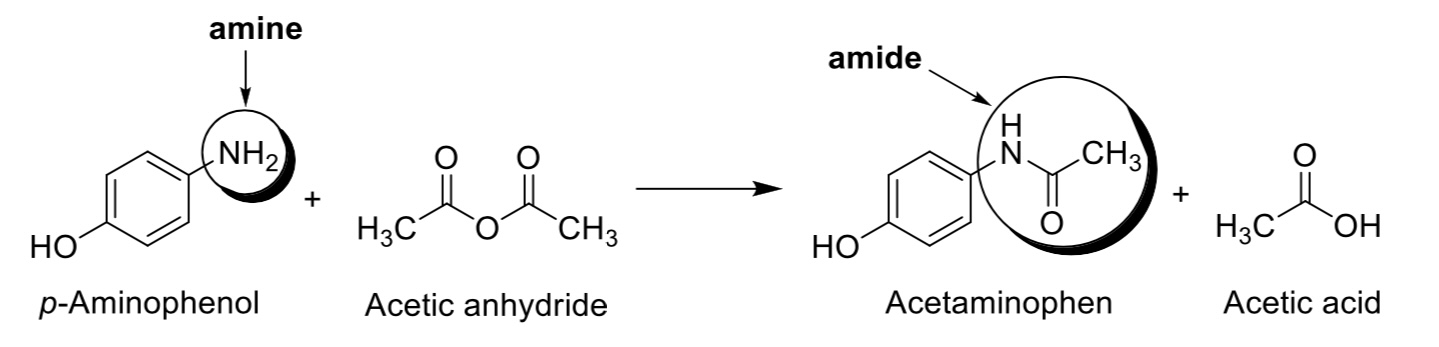
Acetaminophen is formed when the amine group of p-aminophenol (also called 4-aminophenol) is acetylated by acetic anhydride to form an amide functional group. The by-product of this reaction is acetic acid.
After the reaction Acetaminophen is isolated as a crude solid (not pure). This crude solid will be purified using recrystallization. Recrystallization is a purification method that involves dissolving a solid and then causing it to recrystallize (precipitate as a crystal solid) from the solution.
In a typical recrystallization procedure, the crude solid is dissolved by heating it in a minimal amount of solvent (termed recrystallization solvent). The hot solution is then cooled to room temperature and then put in an ice-water bath, whereupon crystals solidify out of the saturated solution. The crystals obtained after recrystallization are purer than the crude solid because most of the impurities remain dissolved in the cold solution.